Reports of research work funded by grants prior to 2012
University of Otago Wellington, School of Medicine and Health Sciences
Analysis of hepatocyte insulin signalling via siRNA-based gene silencing
V Besic, MT Hayes and RS Stubbs
Department of Pathology and Molecular Medicine
Type 2 diabetes (T2DM) is a complex disease resulting from increasing insulin resistance and diminished pancreatic β-cell insulin secretion. The failure of insulin to regulate glucose production at the liver is a critical step in the progression to T2DM. This grant and others awarded in the past have allowed us to investigate insulin signalling at the level of the liver.
We have been investigating the mechanisms behind insulin signalling and its clearance from the circulation at the liver by studying the insulin receptor, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) and insulin degrading enzyme (IDE). Insulin receptor binds and transmits the insulin signal. CEACAM-1 controls the internalisation of the insulin receptor-insulin complex. IDE is the main enzyme which breaks down the active insulin molecule.
Insulin resistance is associated with diminished insulin clearance at the liver. We sought to understand whether the inability of liver cells to clear insulin had an effect on insulin signalling and thus its ability to regulate glucose metabolism. Small interfering RNA were used to restrict CEACAM-1 and IDE protein levels in HepG2 cells. We found that reducing either CEACAM-1 or IDE protein levels did not affect the inhibition of glucose production (gluconeogenesis) after insulin treatment.
Insulin signalling occurs primarily through the insulin receptor. Alternative splicing of the insulin receptor mRNA transcripts produces two insulin receptor isoforms, A and B. These isoforms (IR-A, IR-B) have different signalling functions. Consequently, altered expression in key metabolic tissues has implicated them in the pathogenesis of insulin resistance and T2DM.
In the normal state IR-B mRNA expression predominates at the liver with the ratio of IR-B:IR:A being 8:1. We have found that the ratio of IR-B:IR-A increases from 5:1 to 8:1 in individuals who have a remission of T2DM after Roux-en-Y gastric bypass surgery. This is due to a decrease in the level of IR-A mRNA expression.
Consequently we have used vector technology to overexpress IR-A or IR-B in an in vitro liver cell model (HepG2 cells). We investigated what affect this had on insulin signalling. We measured the phosphorylation of Akt, a key component in the control of glucose metabolism in insulin signalling. We also measured levels of PCK1 mRNA, a rate limiting enzyme in the process of glucose production. Insulin decreases PCK1 mRNA levels thus inhibiting gluconeogenesis.
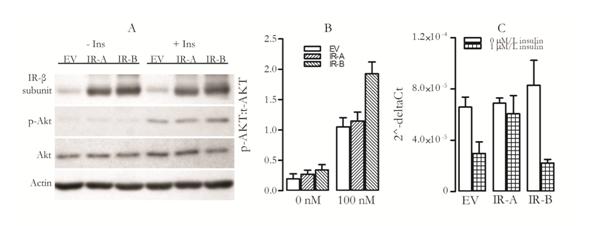
Figure 3. Akt phosphorylation and PCK1 inhibition in HepG2 cells overexpressing IR-A or IR-B after treatment with insulin. All cells were starved for 24 hours prior to insulin stimulation; empty vector was used as control. A) Cells were treated with 100 nM/l insulin for 10 min after which phosphorylated Akt levels were estimated by western blot, B) Quantification of western blot, phosphorylated Akt was normalized to total Akt and C) Cells were treated with 1µM/L insulin for 11 h after which RNA was extracted and RT-qPCR was performed to assay levels of PCK1 mRNA. Bars and error bars represent mean+ S.E.M.
We found that HepG2 cells overexpressing IR-B have higher Akt phosphorylation than cells treated with empty vector (Figure 3 B). Overexpressing IR-A did not significantly affect Akt phosphorylation (Figure 3 B). This data suggests that the IR-B isoform has a greater role in insulin induced metabolic signalling than IR-A isoform.
The ability of insulin to suppress gluconeogenesis was investigated by analysing mRNA expression of the enzyme PCK1. PCK1 mRNA levels were reduced 2.3 fold in empty vector control cells after insulin treatment (Figure 3 C). This reduction was greater in cells overexpressing IR-B isoform (3.1 fold) but was lower in cells overexpressing IR-A isoform (1.4 fold) (Figure 3 C). This data suggests that increasing the level of IR-A isoform diminishes insulin’s ability to inhibit glucose production and is consistent with the loss of control of gluconeogenesis seen in patients with diabetes and their consequent high fasting glucose levels.
Taken together these data confirm that IR-B isoform is essential in regulating glucose metabolism at the liver. Additionally, skewing the B:A ratio towards IR-A isoform decreases the ability of insulin to inhibit gluconeogenesis. Consequently, the altered B:A ratio seen in individuals with T2DM presents a possible mechanisms that is behind the insulin resistance at the liver leading to increased glucose production and ultimately T2DM. The support provided by the WMRF has been crucial in supporting the above work which is a major component of a PhD program. It has given rise to novel information that will be the subject of an international publication.



